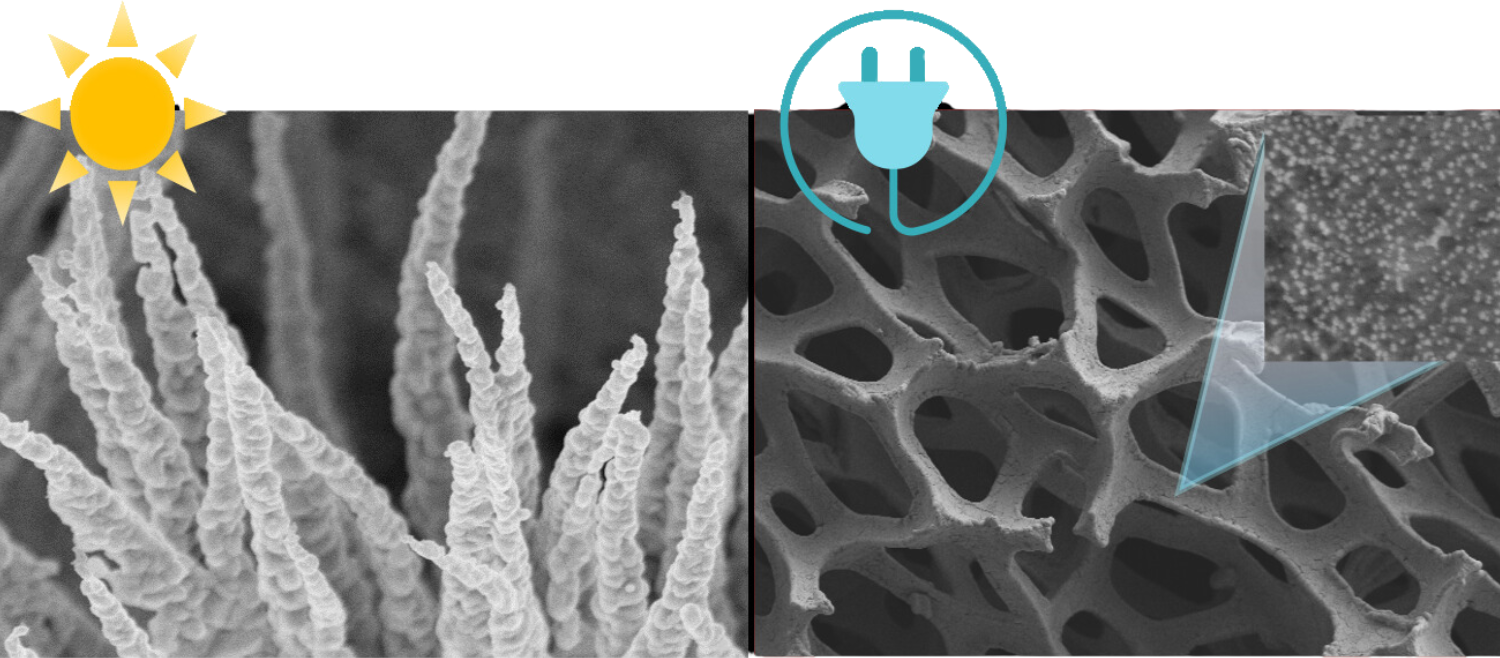
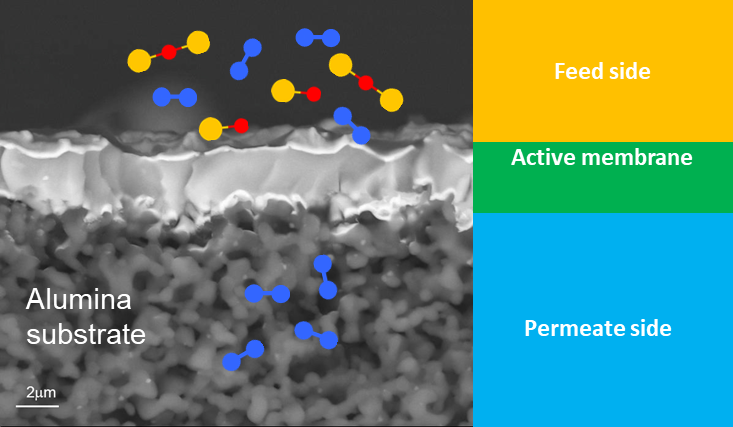
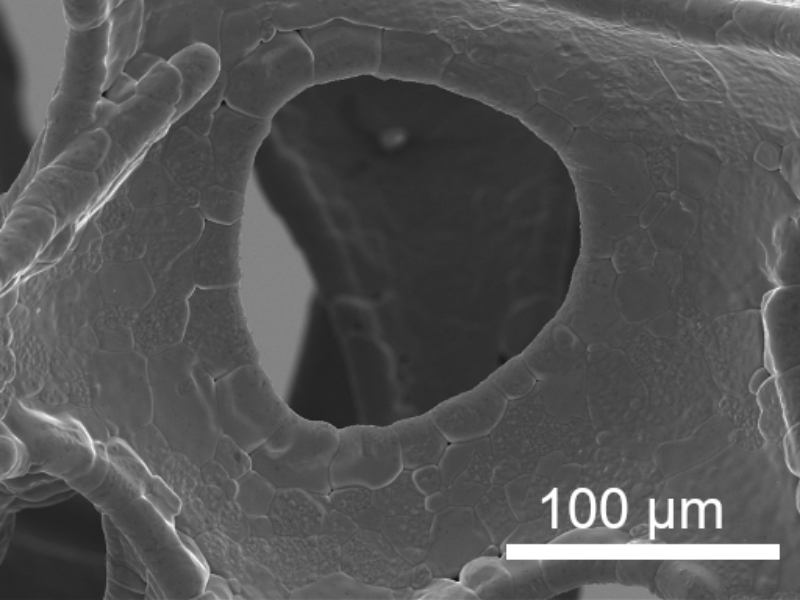
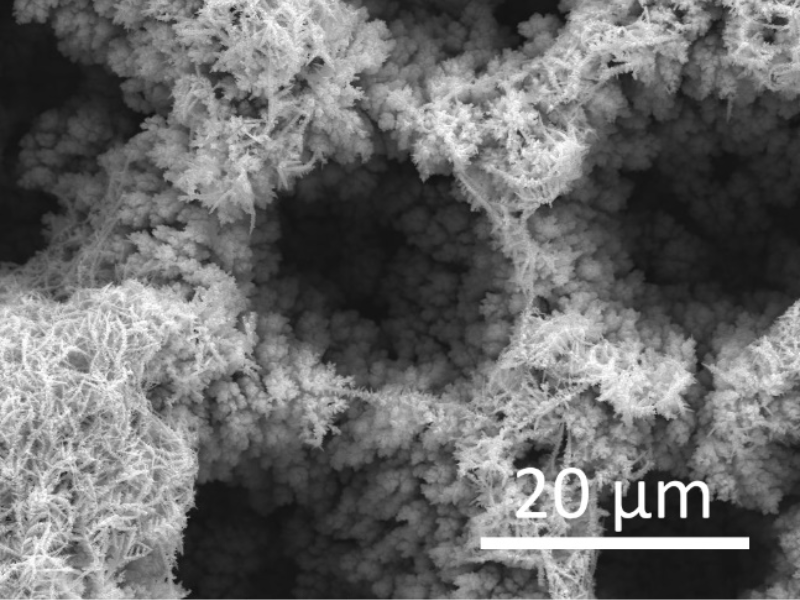
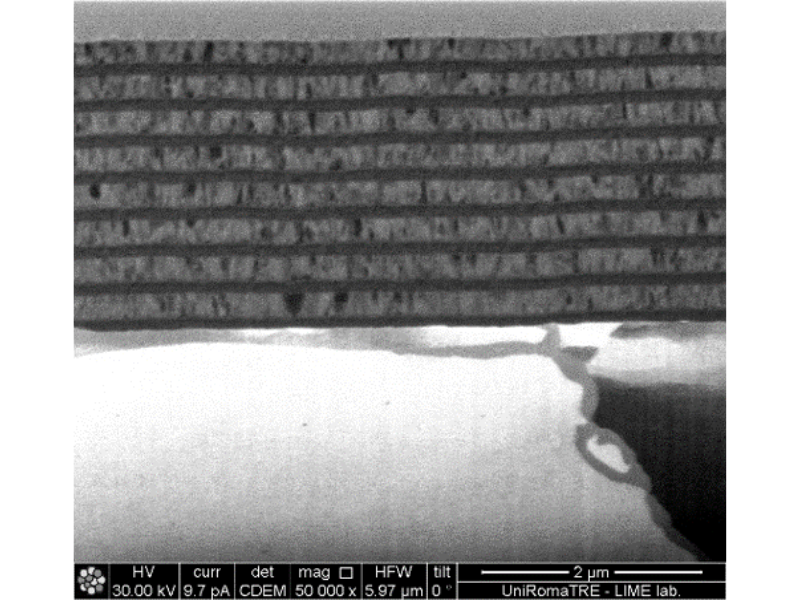
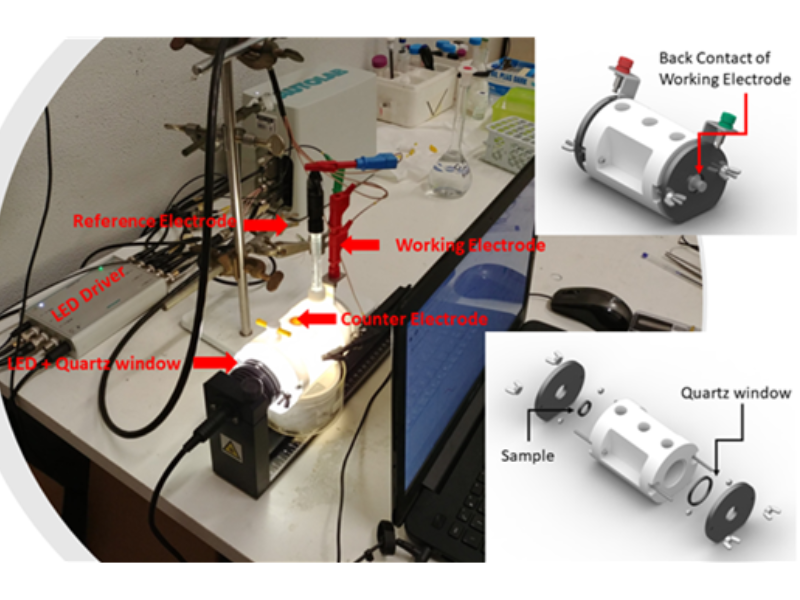
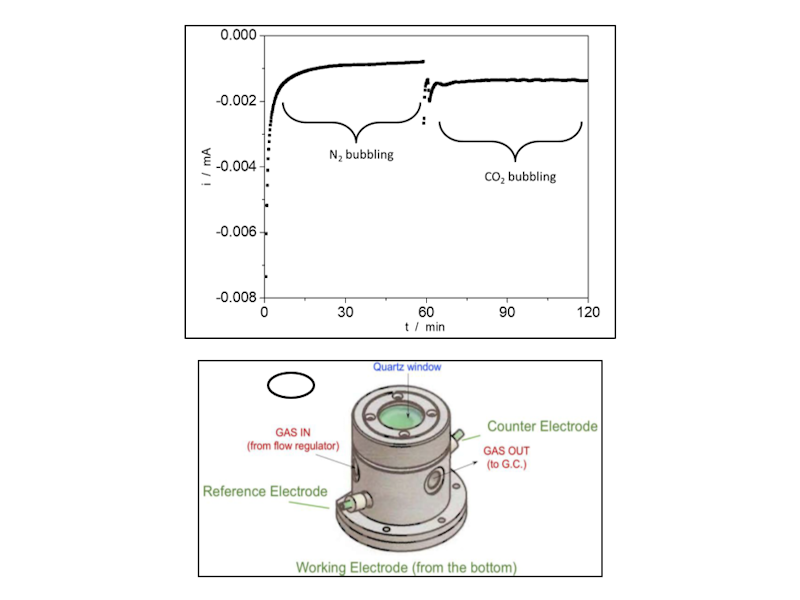
The photoelectrocatalytic production of hydrogen is a promising method that uses sunlight and electrochemical reactions to generate hydrogen fuel. It combines photochemistry and electrochemistry, utilizing light energy to produce hydrogen from water. Recent advances include the development of more stable and efficient materials, nanostructured materials for better light absorption and surface area, and tandem systems that use different semiconductors to harness a broader sunlight spectrum. ICMATE researchers focus on creating novel catalysts for photocatalytic and electrocatalytic hydrogen production, improving water splitting efficiency, and enhancing catalyst durability using materials like metal oxides and functional coatings.
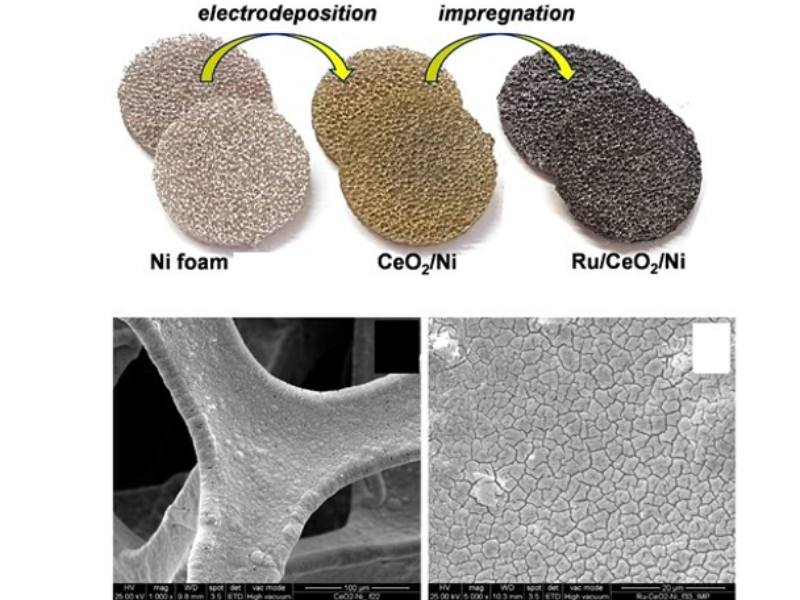
The research is also active in producing hydrogen efficiently, reliably, and cost-effectively, which is crucial for sustainable development, addressing challenges like reducing overpotential, ensuring efficiency under renewable energy sources, and minimizing the use of noble metals in electrocatalysts. Key efforts include selecting high-surface-area conductive substrates, optimizing catalyst amounts using chemical and electrochemical methods, employing novel synthesis techniques for unique compositions, and thoroughly characterizing the catalysts’ properties to set performance benchmarks for hydrogen production.